

The orbitals in the solid are constructed in the same way as those in a molecule, but they apply to a crystal rather than a molecule, so they are called crystal orbitals rather than molecular orbitals. The explanation, which is called band theory, applies the concepts presented in the molecular orbital discussion in Chapter 6 to a very large number of orbitals. Introduction to Band Theory The molecular orbital description of the delocalized electrons in metallic bonds provides a more complete picture of metallic bonding as well as an explanation for the electrical conductivity of metals.

When the unit cell is repeated in all three directions, it generates the entire crystalline lattice. The simplest portion of the lattice that makes up the repeating unit is called the unit cell. The pattern of the array is called the crystal lattice, and the individual positions are called lattice sites. Crystalline solids are orderly, repeating, 3-D arrays of particles, which can be atoms, ions, or groups of atoms, such as polyatomic ions or molecules. The unit cell is the smallest repeat unit of the crystalline lattice that generates the entire lattice with translation. In this section, we define the unit cell and discuss how it is packed with atoms. Our study is simplified because, instead of studying the positions of the enormous number of particles that constitute the entire crystal, we need study only the small number of particles that comprise a unit cell. 8.1 Unit Cells Introduction However, the long range order that characterizes crystalline solids means that there is a small repeat unit, called the unit cell, that can be used to generate the entire crystal.
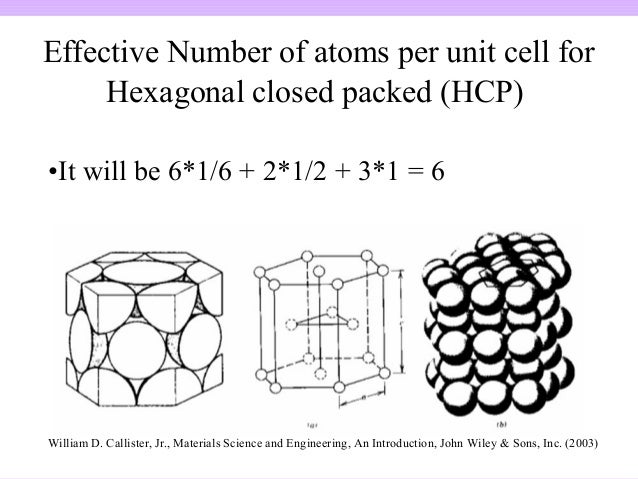
Thus, studying the solid state could be a formidable task. Even a small crystal contains millions and millions of particles. This chapter is devoted to the study of crystalline solids. Table salt and sugar are two common examples of crystalline solids.
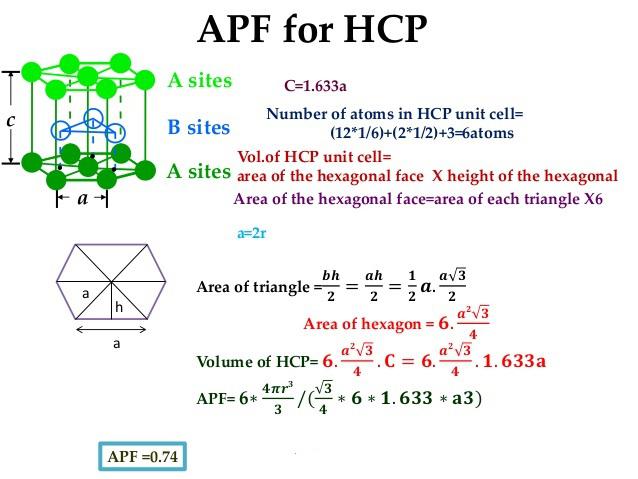
If the order exists throughout the entire solid (long range order), the solid is said to be a crystalline solid. If the order is over short distances only (local order), the solid is an amorphous solid. Therefore, the atomic packing fraction for HCP is equal to the 0.74.Chapter 8 – Solids Introduction Solids are characterized by an orderly arrangement of their particles. For the hexagonal close packing structure$(\text=0.74\] Hint: The volume of the unit cell can be determined by taking the product of the area and height of the cell.


 0 kommentar(er)
0 kommentar(er)
